|
|
|
|
|
Research Projects:: Formation and Stability of Zwitterionic Amino-Acid Enolates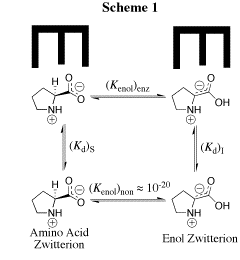
There have been many qualitative studies of the carbon deprotonation of L-amino acid residues in peptides and proteins. For example, racemization of amino acid residues and exchange of the α-amino proton for labeled hydrogen from solvent have both been observed during the hydrolysis of proteins in acidic and basic solution. Amino acid residues at proteins have been shown to racemize faster than amino acid monomers; and, the relative rates of racemization of different amino acid residues at proteins have been reported. Finally, the levels of accumulation of D-aspartic acid in the brain, tooth enamel, bones and lens protein have been determined and shown to provide a rough measure of the age of these tissues. By comparison, when we began our work in this area there were no quantitative determinations of the carbon acid pKa of the α-amino protons of amino acid residues at peptides or proteins. These carbon acidities are required for any full characterization of the chemical reactivity of these important compounds. They are relevant to the mechanism of racemization of amino acid residues in tissue, fossils, marine sediments and agricultural produce. They are also of significant chemical interest, because of the insight that they would provide into the effect of the different functional groups present in peptides and proteins on the acidity of α-carbonyl protons. 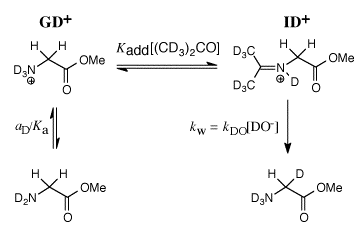
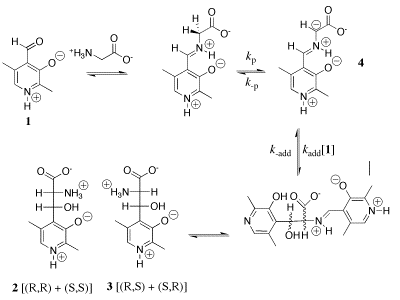
Our work in this area has provided a thorough characterization of the acidity of the α- amino protons of glycine and derivatives of glycine including di-and tripeptides of glycine. We have also determined the acidity of α-amino protons of proline methyl ester. These data are important in their own right; and they have provided important insight into the mechanism for catalysis of proton transfer reactions of α-amino acids. See (a) Journal of the American Chemical Society, 122, 9373-9385 (2000) and (b) Journal of the American Chemical Society, 124, 8251-8259, (2002). (1) Scheme 1 shows a comparison of nonenzymatic enzyme-catalyzed deprotonation of proline, using our data for the thermodynamic barrier to enolization. The principal barrier which must be lowered to achieve the ca. 19 kcal/mol stabilization of this transition state effected by enzymes such as proline racemase is the 27 kcal/mol thermodynamic barrier to conversion of the amino acid zwitterion to the zwitterionic enol (Kenol = 10-20, Scheme 1). This large barrier to keto-enol tautomerization of proline zwitterion in water can be reduced at an enzyme active site provided the enzyme binds the enol zwitterion intermediate more tightly than it binds the substrate amino acid zwitterion, so that (Kd)S >>(Kd)I (Scheme 1). Our work has suggested an important mechanisms by which such selectivity for binding of the intermediate of the reaction catalyzed by proline racemase may be achieved. (2) We have to reported extraordinarily efficient catalysis of deprotonation of the α-amino carbon of glycine methyl ester by the simple ketone acetone and shown that this catalysis is the result of a 107-fold larger acidity constant KCH for carbon deprotonation of the iminium ion adduct IH+ (pKCH = 14) than for deprotonation of N-protonated glycine methyl ester GH+ (pKCH = 21). See Journal of the American Chemical Society, 123, 7949-7950 (2001). (3) The chemistry of pyridoxal 5'-phosphate (PLP) is thought to be well understood, and we expected that the PLP analog 1 would be an effective catalyst of the deuterium exchange reaction of glycine. We were therefore surprised when 1H-NMR analysis of the reaction of 10 mM 1 with 100 mM glycine in D2O at pD 7.0 and 25 C showed the loss 1 in a clean first-order reaction (kobsd = 4.3 x 10-5 /s) to form an equilibrium mixture that contains 3% of 1 and two isomeric products in a ratio of 2.1:1.0 that were identified as adducts of glycine to 1. There is no detectable (<1%) transfer of deuterium from D2O to glycine or formation of 2'-deoxypyridoxamine from a transamination reaction during the first four halftimes of the reaction of 1. We find instead that the reaction between 1 and glycine gives a quantitative yield of 2 and 3 from a Claison condensation reaction (Scheme 2). The Claisen condensation reaction between 1 and glycine escaped characterization in earlier work, because of its strangeness in comparison to textbook PLP-catalyzed reactions. Water is a poor solvent for Claisen condensation reactions, because this solvent is moderately acidic and will rapidly protonate basic enolates of simple carboxylic acid derivatives. The formation of 2 and 3 in good yields from addition of the glycine enolate 4 to 0.1 mM 1 in water is a consequence of the unprecedented large selectivity of 4 for addition to 1 in a protic solvent that contains 50 mM of a buffer acid of pKa 7. [In Preparation]
|
| |||||||||
All material © John Richard 2003-2004 | Website Developed by Iserloh Design | ||||||||||